Rescan-line-sted
This project is maintained by AndrewGYork in the York lab, and was funded by Calico Life Sciences LLC
Research article
Note that this is a limited PDF or print version; animated and interactive figures are disabled. For the full version of this article, please visit https://andrewgyork.github.io/rescan_line_sted
Line-rescanned STED microscopy is gentler and faster than point-descanned STED microscopy
*Institutional email: agy+linerescan@calicolabs.com; Permanent email: andrew.g.york+linerescan@gmail.com; Group website: andrewgyork.github.io
Abstract
Stimulated emission depletion (STED) [Hell 1994] substantially improves the resolution of point-scanning confocal microscopy, at the cost of reduced emission signal and massively increased illumination dose. The resulting tradeoffs between resolution, speed, signal-to-noise ratio, and photodamage can be frustrating, especially for live samples. We describe a gentler, much faster alternative technique based on a combination of STED with line-scanning confocal microscopy [Wolleschensky 2006], analog structured illumination microscopy [York 2013, Roth 2013, De Luca 2013], and multi-image fusion [Ingaramo 2014].
Intended audience
Engineers and physicists familiar with modern microscopy techniques, including stimulated emission depletion and structured illumination. Instead of equations, we include the complete Python code which generates each figure, to facilitate reproducing and exploring our results.
Peer review status
Pre-print published April 7, 2017 (This article is not yet peer reviewed)
Cite as: doi:10.5281/zenodo.591251
Introduction
STED improves microscope resolution at the expense of speed and gentleness
The STED technique uses an intense depletion ‘doughnut’ (Fig. 1a, green) to shrink the fluorescence excitation region (Fig. 1b, dotted blue) of a point-scanning confocal microscope. As the illumination is scanned across the sample (Figure 1a, 1b, gray), a fluorophore endures many excitation-depletion cycles before and after it is allowed to briefly emit signal (Figure 1b, solid blue). If the STED doughnut improves lateral resolution by a factor of \(R\) (Figure 1e), the 2D area of the emission region decreases by roughly \(R^2\) (reducing emissions per scan and emissions per second) and the number of scan positions (Figure 1a and 1b, gray) must increase by a factor of \(R^2\) to satisfy Nyquist sampling (Figure 1c, red). At least one full-intensity depletion pulse must be delivered at each of these finer scan positions to maintain \(R\), and of course the depletion pulse intensity grows rapidly with \(R\).

| Depletion: | (peak fluence per depletion pulse, in saturation units) | |
| Excitation: | (peak fluence per excitation pulse, in saturation units) | |
| Scan density: | (scan positions per excitation PSF FWHM) | |
| Exposure time: | (excitation/depletion pulse pairs per scan position) | |
| Shape: | (2D point-scan, or 1D line-scan) |
Together, these factors mean STED requires a massive illumination dose and slowly yields a small signal compared to standard confocal, even for moderate \(R\), even when optimally tuned. This makes STED an appealing improvement to confocal microscopy in some cases, but not a general-purpose replacement. Can we do better?
Instead of a doughnut, what if we try an eclair? Stretching our excitation and depletion patterns into lines
[Curdt 2013,
Heller 2013]
massively parallelizes the signal emission area, and greatly reduces the number of excitation-depletion cycles each fluorophore undergoes before and after emission (Figure 1 with Shape: Line selected; note the reduced number of scan positions). The same peak depletion intensity yields the same resolution improvement for both point-STED (Fig 1 with Shape: Point selected) and line-STED (Fig. 1 with Shape: Line selected), but line-STED’s total emission, emission rate, and number of scan positions scale much more favorably, like \(R\) rather than \(R^2\), suggesting that line-STED is gentler and faster. Is it? How should we judge?
Results
Line-scanning STED is gentler than point-scanning STED
A gentler technique gives higher image quality for the same photodose. Unfortunately, the simplest comparison (changing Figure 1 from Shape: Point to Shape: Line) isn't fair or conclusive; line-STED decreases excitation and depletion dose compared to point-STED, but also reduces emissions per molecule and badly degrades resolution perpendicular to the scan direction.
How can we compare fairly? By appropriate tuning, we can compare line vs. point STED at equal photodose. To compare image quality, we simulate a standard method to handle anisotropic resolution: acquire images from multiple scan directions [Schubert 2013, Wu 2013], and fuse these measurements into a single high-quality image via iterative deconvolution [Ingaramo 2014].

| Point-STED resolution: | (improvement compared to the diffraction limit) | |
| Image: | (choice of resolution targets) | |
| Line-STED scan type: | (see section Rescanned line-STED speed) |
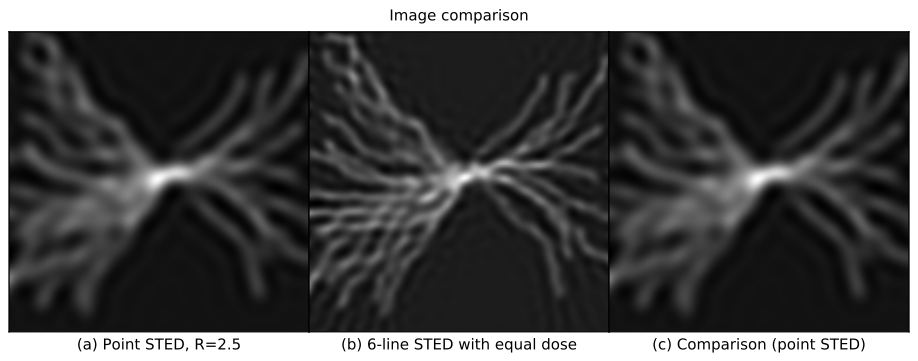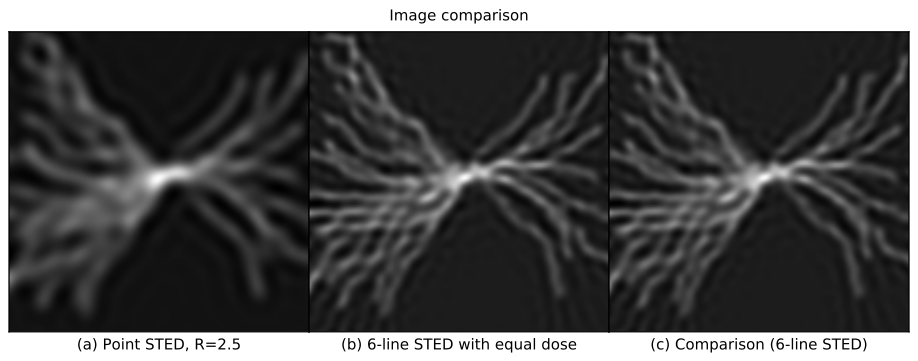
This (admittedly complex) comparison confirms our suspicion: line-STED is gentler than point-STED. Qualitatively, a point-STED image (Figure 2a) is lower quality than a line-STED image using the same photodose (Figure 2b) for any choice of Point-STED resolution; an animated overlay (Figure 2c) emphasizes these qualitative differences. Quantitatively, line-STED images are more accurate than point-STED images, yielding a larger range of spatial frequencies measured with low error (Figure 2d), and similar or lower error levels at each of these frequencies (Figure 2e).
Note that point-STED resolution is laterally isotropic, but multi-orientation-fusion line-STED resolution is not. The image of a pointlike object (called the point spread function or PSF) is round for point-STED (Figure 2f), but elliptical with varying orientations for each line-STED scan direction (Figure 2g). This causes a star-shaped pattern in line-STED's error vs. spatial frequency (Figure 2d, right panel), meaning fine structures will be more visible at some orientations than others. In the worst-case directions (Figure 2d,e, dotted blue line), line-STED resolution is similar to point-STED with the same photodose, but in the best-case directions (Figure 2d,e, solid blue line), resolution is approximately twice as good. Selecting Shape: Rings in Figure 2 highlights this difference qualitatively (Figure 2c); even for the least favorable orientations, line-STED reveals finer rings than point-STED.
Rescanned line-STED is faster than descanned point-STED, multipoint-STED, and descanned line-STED
A STED microscope delivers at least one excitation/depletion pulse pair per scan position, and consecutive scan positions are typically separated by at least two fluorescence lifetimes (which helps avoid confusion about which scan position produced which detected photoelectron). Typical fluorophore lifetimes range from 0.5 - 10 nanoseconds, suggesting a fundamental limit to STED speed on the order of ~\(10^8\) scan positions per second. The more scan positions a STED microscope requires to form a useful image, the more its speed suffers from this limit, especially if multiple pulses are required at each scan position to improve signal-to-noise ratio (SNR).
Point-STED speed
Single-point STED (Figure 3 with Imaging method: Descan point) can use nearly instantaneous scanning and nearly ideal detectors
[Schneider 2014],
leaving the number of scan positions (Figure 3d) as its primary speed limit. This limit scales poorly for point-STED, like the product of \(R^2\) and the two lateral image dimensions (compare Figure 3d for STED resolution: 1.00 vs. STED resolution: 3.00, and also for Field of view: 1 by 1 vs. Field of view: 2 by 2). This limit still allows 10-1000 frames per second (for sufficiently small fields of view), but not with usable SNR in a single frame. Multiple pulses per scan position are always used to improve SNR, reducing point-STED's effective speed well below video rate unless the sample is extremely bright, sparse, and small
[Westphal 2008,
Schneider 2014].
| Imaging method: | (STED) | |
| STED resolution: | (compared to the diffraction limit) | |
| Image: | (choice of test image) | |
| Field of view: | (repetitions of test image) | |
| Orientations: | (number of scan directions, line-STED only) |
Multipoint-STED speed
Parallelizng STED via multiple excitation/depletion points
[Bingen 2011,
Yang 2014,
Bergermann 2015]
can greatly reduce the number of scan positions (compare Figure 3d with Imaging method: Descan point vs. Imaging method: Multipoint). The number of scan positions still scales like \(R^2\) (compare Figure 3d with STED resolution: 1.00 vs. STED resolution: 3.00), since the step size must satisfy Nyquist sampling and excitation points are typically separated by at least the width of the emission PSF to avoid crosstalk. However, the number of scan positions can be independent of the field of view, potentially an enormous speedup (compare Figure 3d with Field of view: 1 by 1 vs. Field of view: 2 by 2).
Unfortunately, multipoint STED is bottlenecked by existing array detectors. Highly parallel multipoint STED implementations [Yang 2014, Bergermann 2015] acquire \(10^2\)-\(10^3\) camera frames (Figure 3e) per STED image, and spread the signal from each STED point across \(10^2\)-\(10^3\) detector pixels, massively decreasing effective readout rates and increasing effective read noise by at least an order of magnitude. Despite its promise to increase speed, this bottleneck means that multipoint STED is currently slower and more phototoxic than single-point STED (compare [Schneider 2014] vs. [Bergermann 2015]).
Descanned line-STED speed
Existing eclair-based line-scanning STED techniques
[Curdt 2013]
offer a qualitatively similar tradeoff to multipoint STED (compare Figure 3d,e with Imaging method: Multipoint vs. Imaging method: Descan line), decreasing scan positions compared to point-STED at the expense of a large number of camera exposures, and spreading the signal from a single scan point across multiple detector pixels. However, descanned line-STED gives several important quantitative advantages over multipoint STED. Since the image of the excitation line (Figure 3a) is descanned onto the camera, only lines within a few emission PSF widths of the center of the chip must be read for each exposure (Figure 3b), which is much faster than reading the whole chip (especially for sCMOS detectors). Several tens of detector pixels are summed to produce each logical pixel rather than hundreds or thousands, giving a substantial decrease in read noise compared to multipoint-STED; this means that the gentleness advantage of line-STED over multipoint-STED is even greater than Figure 2 suggests. Also note that line-STED requires substantially less energy per depletion pulse than point-STED, since the illuminated area is one or more orders of magnitude smaller, depending on the field of view.
The primary drawback of line-STED vs. point-STED is the need for multiple scan directions (Orientations: in Figure 3), but fortunately, scan orientation can be switched extremely rapidly. The number of line-STED scan positions scales like the product of \(R\) (STED resolution: in Figure 3) and the largest lateral image dimension (compare Figure 3d for Field of view: 1 by 1 vs. Field of view: 2 by 2), and the number of scan directions (which also scales roughly like \(R\); compare Figure 2g for several values of Point-STED resolution:).
Rescanned line-STED speed
Line-STED improves further if we rescan the emission instead of descanning (compare Figure 3d,e with Imaging method: Descan line vs. Imaging method: Rescan line). Rescanning can be implemented similarly to rescan confocal/OPRA
[Roth 2013,
De Luca 2013]
or instant SIM
[York 2013]; in either case, rescanning enhances the already promising speed and gentleness advantages of descan line-STED.
Rescanning uses only one camera exposure per scan orientation (Figure 3e) and reads only one camera pixel per logical pixel (Figure 3b,c), which substantially improves the detector's effective readout rate. This also substantially improves read noise, further improving both speed and gentleness. Rescanning also gives a "free" resolution improvement, especially at low depletion power (compare Figure 3c for Imaging method: Descan line vs. Imaging method: Rescan line, with STED resolution: 1 and Image: Rings selected; also compare Figure 2 for Line-STED scan type: Descanned vs Line-STED scan type: Rescanned, with Point-STED resolution: set to 1.00x or 1.50x). Since a gentler technique gives higher image quality for the same photodose, this is yet another reason rescanning is gentler than descanning. Note that rescanning gives a strict improvement compared to descanned line-STED; there are no drawbacks apart from additional optics.
We've seen that rescanned line-STED is faster and gentler than descanned line-STED, which in turn is faster and gentler than multipoint STED. Finally, compare Figure 3d,e for Imaging method: Descan point vs. Imaging method: Rescan line, and note that rescan line-STED reduces the number of scan positions by an order of magnitude or more, in all cases.
Discussion
Rescanned line-STED promises a strict improvement vs. point-STED, mulitpoint-STED, and descanned line-STED: improved image quality for the same photodose (Figure 2), acquired faster (Figure 3), in a way that's not bottlenecked by existing cameras and doesn't require excessively high laser pulse energies. Care must be taken with both the hardware and software to deliver on this promise, particularly the integration between acquisition and image fusion post-processing, but this is already true of modern microscopes like the Zeiss Airyscan or the dual-view SPIM.
It's interesting to note that although line-STED gives strictly less information per emission than point-STED, it gives strictly more information for a given photodose (Figure 2). We speculate that this advantage of lines vs. points (or eclairs vs. doughnuts) may extend to other contexts (e.g. particle tracking [Balzarotti 2017]). Perhaps the story does not end at lines and points, and the study of a wider variety of illumination shapes [Chakrova 2015] could identify even more optimized approaches [Shechtman 2014].
Appendix
Additional details and discussion can be found in the Appendix, which is also referenced via hyperlinks throughout this article.
Updates
Egner et al. described "tomographic STED microscopy" at the 2017 Focus on Microscopy conference. Tomographic STED was conceived and demonstrated independently from this article, and differs in many important ways from rescanned line-STED, but still beautifully experimentally demonstrates several of the principles we simulate here. Their method is a type of point-descanned STED using a 1D depletion pattern, which enlarges the emission region at the cost of anisotropic resolution. They reduce anisotropy by merging multiple orientations of the 1D depletion pattern via iterative deconvolution, yielding a single high-quality image. Their method improves both speed and gentleness compared to a standard depletion "doughnut", and the hardware is sufficiently similar to existing descanned point STED that it promises to be straightforward for others to implement.